Team develops model to determine stability of gas hydrates
Comprehensive model can better predict how gas hydrates and methane stabilization could ease global warming
Department of Energy
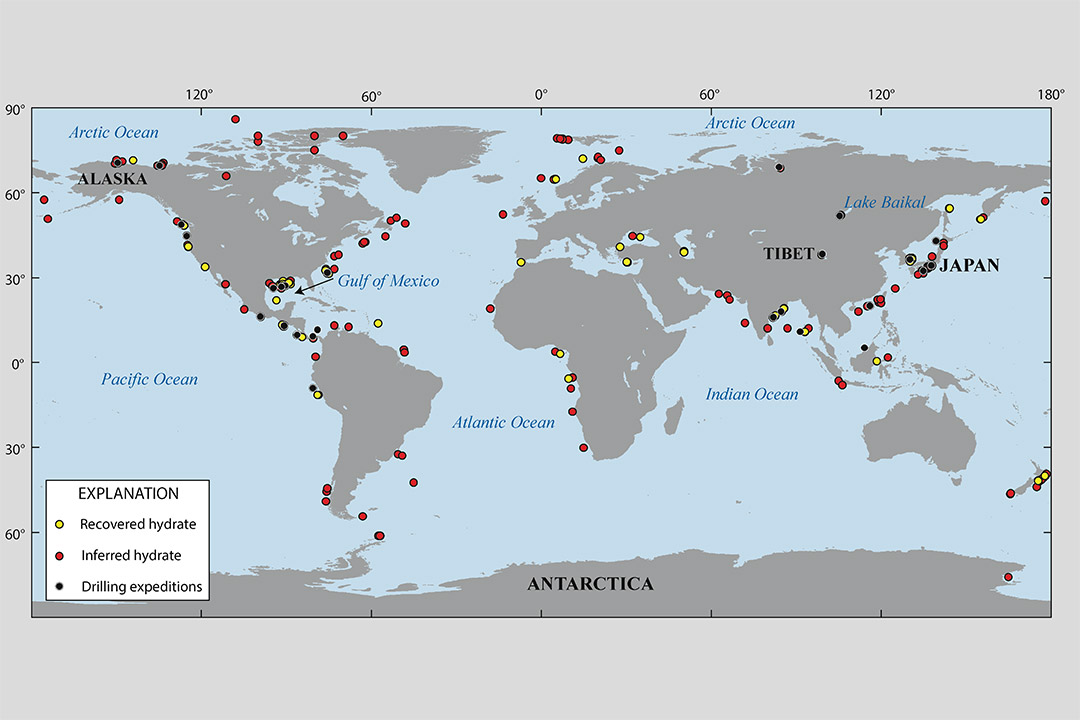
This Department of Energy map shows locations where gas hydrates have been discovered, where gas hydrates is inferred to be present on the basis of seismic data, and where drilling expeditions have been completed in permafrost or deep marine environments, all leading to recovery of gas hydrates.
Natural gas-hydrates—crystalline compounds of gas molecules—are found in permafrost and marine sediments. While these gas hydrates can be used as alternative energy resources, they also pose a danger in terms of global warming, if catastrophic release of gas into the atmosphere was to take place.
Patricia Taboada-Serrano.
Patricia Taboada-Serrano and Yali Zhang, researchers at Rochester Institute of Technology, developed a comprehensive model to better validate location of gas-hydrate deposits in marine sediments. This information can be used to assess stability of the deposits, and the model can also provide predictive information about destabilization of those deposits due to variations in temperature.
The new model could benefit scientists who measure global climate patterns because they have an available model that predicts the stability of gas-hydrates more accurately. Some of the energy stored in these compounds is larger than the world-wide conventional natural gas reserves combined, said Zhang.
“The petroleum crisis could be successfully avoided if we can exploit most of the natural gas from gas-hydrate reservoirs in a safe manner, without contributing to global warming,” said Zhang, a researcher in RIT’s Kate Gleason College of Engineering. “Natural gas is considered clean energy, and huge amounts of natural gas are trapped in gas-hydrates. This clean energy is not only attractive to exploit but also a risk to global warming—methane is a potent greenhouse gas. A given volume of methane causes about 28 times more greenhouse gas warming than carbon dioxide.”
Yali Zhang.
The researchers’ findings were published in the paper, “Model for gas-hydrate equilibrium in porous media that incorporates pore-wall properties.” It was recognized recently as a 2020 HOT Paper by the Royal Society of Chemistry and reprinted in a special edition highlighting highly-contributing scientific papers during the year.
Information about the size and gas-content of gas-hydrate reservoirs has been limited in the past. Natural gas-hydrates are found in geographically diverse settings around the globe, either in marine sediments or permafrost environments, some inaccessible because of harsh conditions and the need for equipment that can safely extract specimens. The researchers’ model can be used to better predict deposit structure, properties, and reliability.
Department of Energy
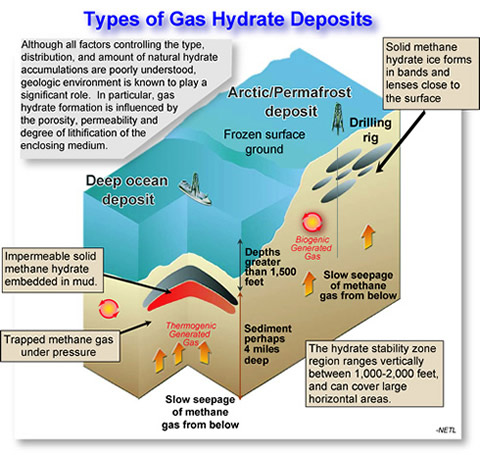
This illustration details the different layers of deposits where gas hydrates could be located and accessed.
“The goal of the work was to develop a predictive model that would be more precise than previous models, and that can be used to truly estimate how much gas is stored in these natural gas-hydrate deposits and how stable these deposits are. Furthermore, we aimed to having the capability to predict what would happen with these deposits if the temperature of the oceans is raised even a little bit, and the pressure changes due to changes of the water levels,” said Taboada-Serrano, an associate professor of chemical engineering in RIT’s Kate Gleason College of Engineering.
The U.S. Department of Energy has been leading exploration and research initiatives into the use of gas-hydrates as a possible alternative energy solution when it was discovered that the total amount of natural gas stored in naturally occurring hydrates is larger than the worldwide conventional natural-gas reserves combined.
“Over the years, people have developed models to try to predict how large these deposits are these ocean sediments. We are seeing ocean temperatures slowly creep up and polar caps melt and reduce in size. This means that we are basically getting to temperatures in which gas-hydrate deposits destabilize. The temperature is becoming high enough to start releasing methane and it may end up in the atmosphere,” said Taboada-Serrano.
Gas-hydrates are structures trapped in sediment, often 1,500 meters below sea level, dormant underneath the polar ice caps, permafrost, and in marine sediments. Challenges include finding and accessing reservoirs in some of the Earth’s inhospitable areas such as the Arctic and developing technologies to verify the stability of the reservoirs and safely enable extractions. Access to these areas is often limited to diving expeditions or coring samples from drilling. These structures are also stable under very specific windows of temperature and pressure. But if the temperature of the ocean rises a little bit, these ice-like structures are going to start to dissolve and start releasing methane into the atmosphere.
“The models developed can be used to predict the equilibrium conditions, specifically, temperature and pressure, kinetic nucleation rate and formation probability of gas hydrates by incorporating sediment properties such as wettability and interfacial energies,” said Zhang, who recently completed her doctoral dissertation in this area under the supervision of Taboada-Serrano. “It is very useful in the sense that many realistic sediment conditions are captured by the model and the model predictions agree with available experimental data very well. Looking forward, I think the model will be widely used by the researchers both in academics and energy industry.”