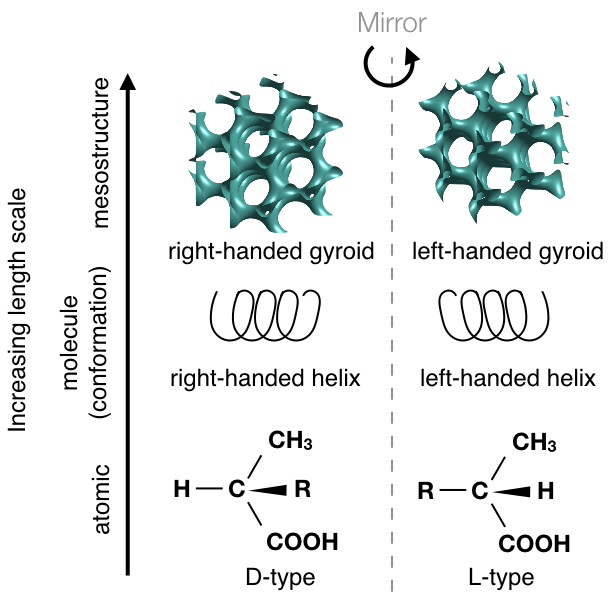
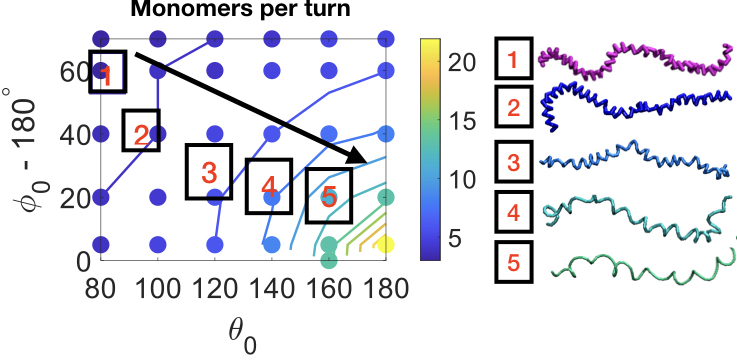
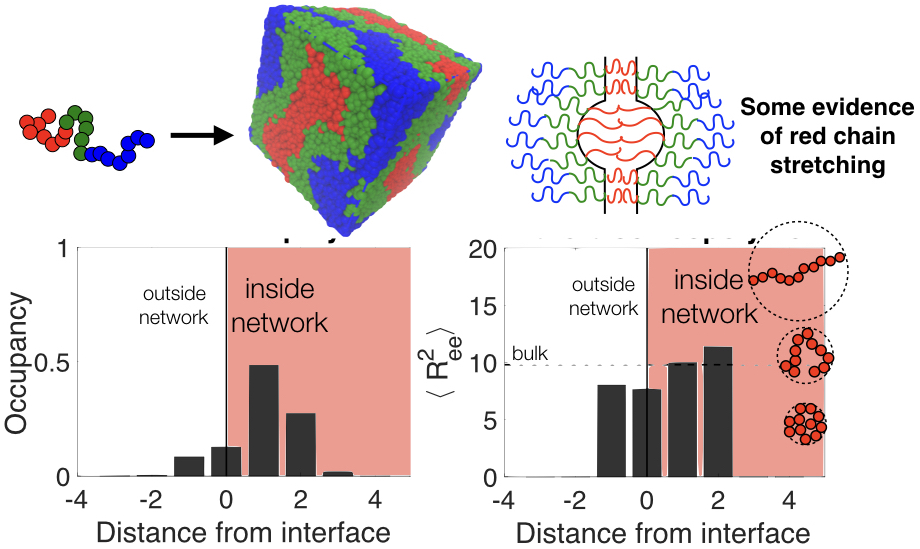
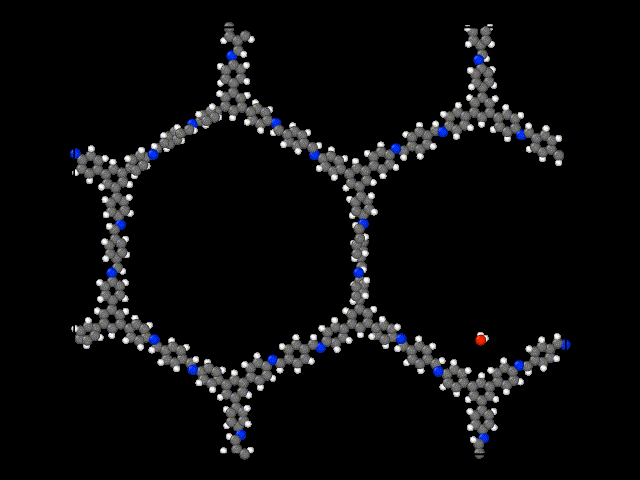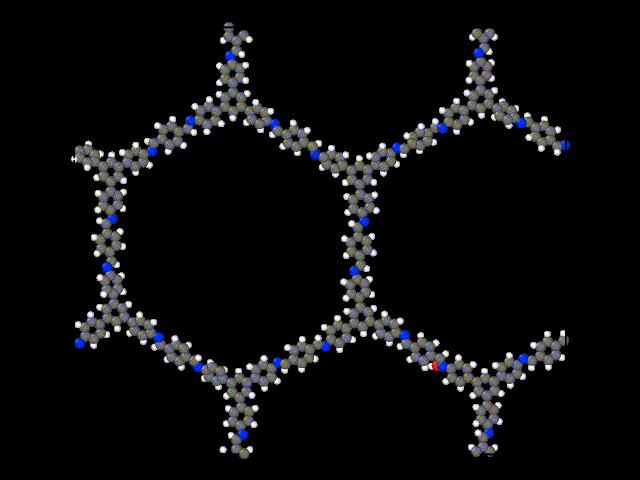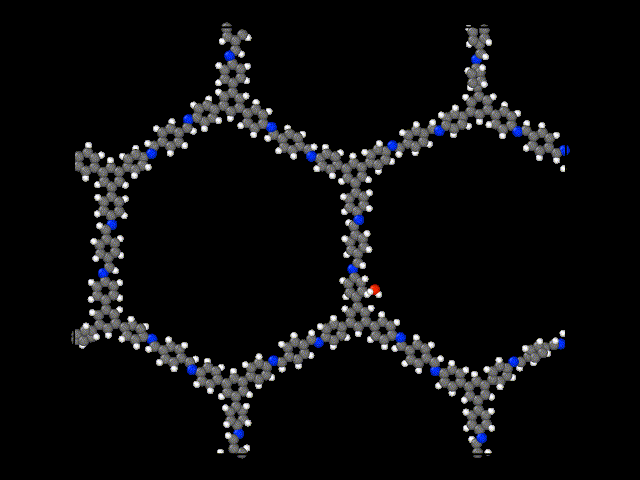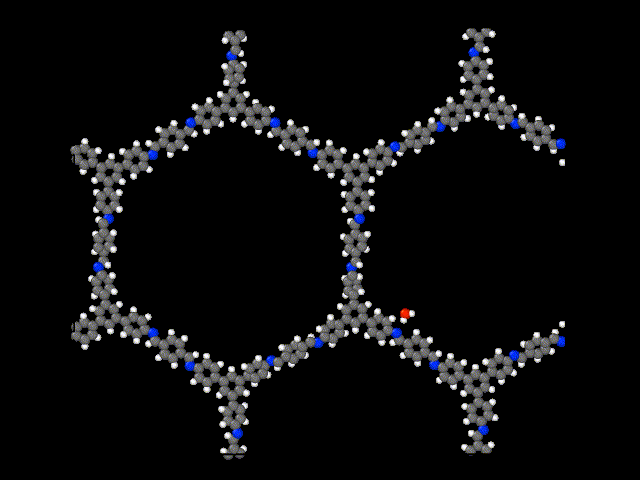
Research
Dr. Padmanabhan's research group works on a bottom-up approach to synthesizing materials with novel structure and novel properties using self-assembly
In other words, we design the molecular building blocks and their chemical interactions, which lead to the complex organization of thousands or millions of molecules into complex structures. Our approach leverages thermodynamics to predict the phase diagram of several workhorse model systems ranging from polymers to colloids to liquid crystals.
Acknowledgements
We gratefully acknowledge the staff and high-performance computing resources provided by RIT Research Computing that enables to do our work.
The Kate Gleason College fund has provided funding from 2017 - 2024 for graduate and undergraduate student researchers.
We acknowledge National Science Foundation for awarding he PI with a CAREER award in 2022 (DMR 2144511) and continued support.
We received funding from the Army Research Labs (DOD Army Materiel Command) from 2020 - 2021 to investigate solvation of 2D polymers.